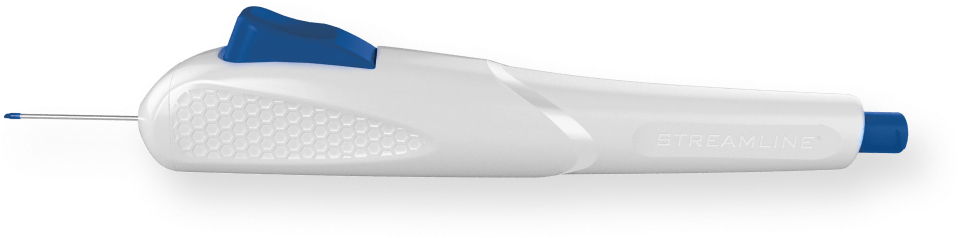
facilitates the lift and stretch of Trabecular Meshwork (TM)
create parallel incisions for clean TM excision.
for optimal interface with the canal of Schlemm

Addresses several clock hours of the conventional outflow system rather than only a specific quadrant4
No foreign bodies left behind4,6
ClickPulse® Technology allows the surgeon to perform the procedure with one hand4
ClickPulse® Technology allows the surgeon to perform the procedure with one hand4
Targets the conventional outflow system4
Targets the conventional outflow system4
Gentle, multipoint applications through the TM into the canal of Schlemm4
Gentle, multipoint applications through the TM into the canal of Schlemm4
Allows constant device visualization4
Allows constant device visualization4
Prepare OVD by expelling air from the OVD syringe. Connect syringe to priming port, then twist to lock5
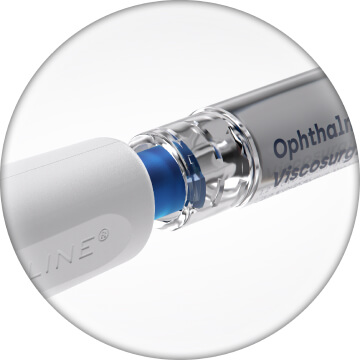
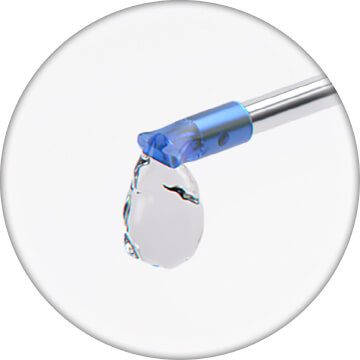
Prime STREAMLINE® by depressing the OVD syringe plunger. Device is fully primed once OVD is seen flowing from the outer sleeve without bubbles. Twist to remove OVD syringe from the priming port before next steps5
Angle patient’s head 45 degrees away from surgeon, and angle microscope 45 degrees toward surgeon

Insert outer sleeve of STREAMLINE® into anterior chamber through existing clear corneal incision (minimum 1.8 mm). Advance outer sleeve past pupil and toward opposite angle5
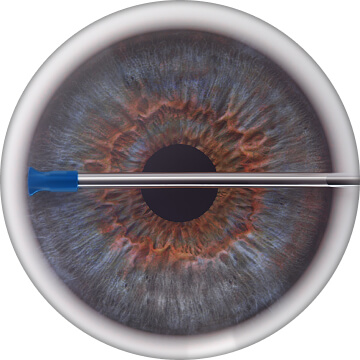
Advance outer sleeve to targeted drainage angle under direct gonioscopic visualization. Locate TM and other angle structures5
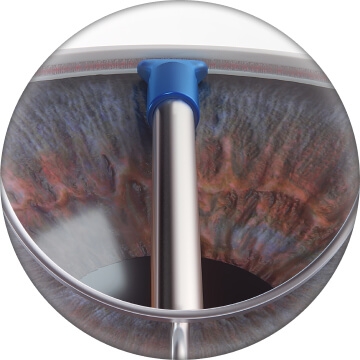
Touch outer sleeve against TM to slightly applanate the TM without indenting eye wall and without losing gonioscopic view of anatomical structures5
Properly orient width of outer sleeve with TM, which aligns inner cannula with the canal of Schlemm5
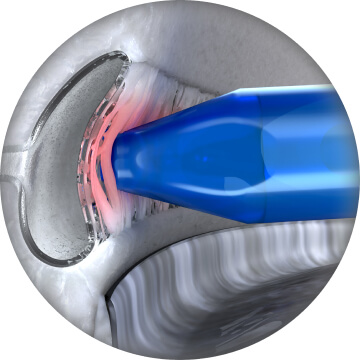
Activate ClickPulse® by fully depressing button in one smooth, continuous motion, while maintaining device position and view of anatomical structures. This creates a precision goniotomy, performs a precision catheterization, and dispenses the OVD5
After 2 seconds, move 1-2 mm away from treated area (remaining under gonioscopic visualization) and lift finger away from button5

Perform additional ClickPulse® applications by moving outer sleeve tip at least 1 clock hour to either side of initial application site. Repeat previous steps (up to 8 total applications)5
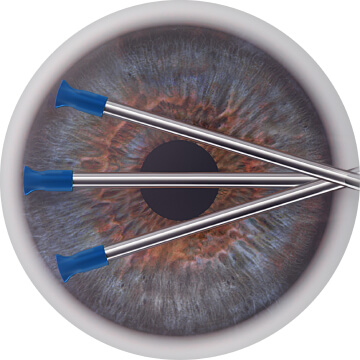
The STREAMLINE® device opens multiple precision goniotomies, then uses OVD to dilate the canal of Schlemm, stretch the TM, and flush the distal collector channels, targeting the conventional outflow system without a permanent implant.4,6,7
A list of recommended brands of OVD can be found in the Instructions for Use.
New World Medical offers training resources here.
For more information about STREAMLINE®, please read the Instructions for Use. You can also call (909) 323-0592 to speak with a representative.
1. Data on file. New World Medical, May 2020. 2. Dorairaj S, Balasubramani GK. Corneal endothelial cell changes after phacoemulsification combined with excisional goniotomy with the Kahook Dual Blade or iStent: a prospective fellow-eye comparison. Clin Ophthalmol. 2020;14:4047-4053. 3. A new MIGS on the block? Cataract & Refractive Surgery Today. November/December 2015 [insert]. Accessed February 9, 2022. https://crstoday.com/articles/2015-novdec/1215_insert3-pdf/ 4. Data on file. New World Medical, February 2020. 5. STREAMLINE® Surgical System [Instructions for Use]. Rancho Cucamonga, CA: New World Medical; REV C, April 2022.
Your rep can discuss the certification process with you